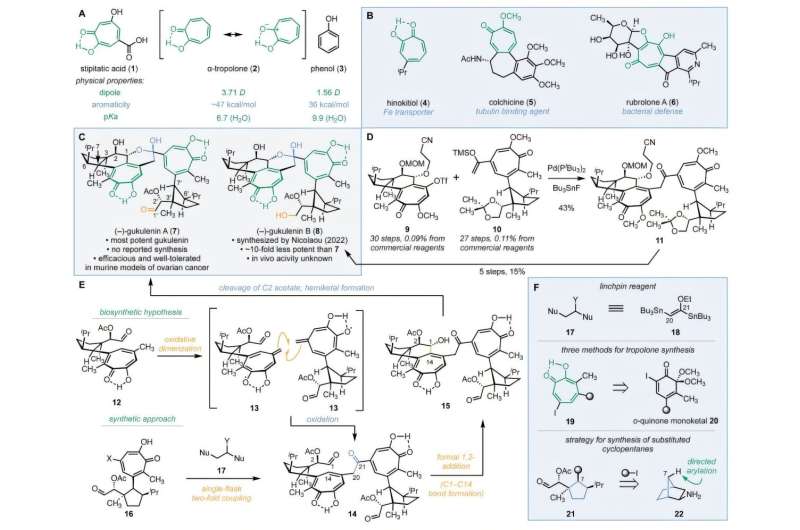
A team of researchers from Yale University has successfully completed the first stereoselective synthesis of the complex natural compound (–)-gukulenin A, which has shown significant cytotoxic effects against ovarian cancer. This breakthrough, reported on November 18, 2025, highlights the compound’s potential as a new therapeutic option in oncology, particularly given its ability to reduce ovarian tumor size by more than 92% in animal studies.
The challenge of synthesizing (–)-gukulenin A lies in its intricate molecular structure. The compound features two α-tropolones, which are seven-membered aromatic rings with strong molecular dipoles, along with ten precise three-dimensional stereocenters. Additionally, it contains delicate chemical groups such as a hemiketal and an aldehyde, making it a formidable target for synthetic chemists. Researchers overcame these synthesis hurdles through a novel three-component assembly strategy, drawing inspiration from naturally occurring biosynthetic pathways.
Innovative Assembly Method
In their approach, the team undertook a three-step synthesis process: constructing two halves of the molecule, linking them together, and closing the final rings. They began with exo-2-norbornylamine, a rigid bicyclic molecule that guided the three-dimensional arrangement needed for synthesis. The researchers developed a new ring-expansion method to convert a six-membered ring into the seven-membered tropolone structure required for the monomers.
The pivotal moment came when the researchers introduced a previously unknown two-carbon linking reagent, identified as (E)-1,2-di(tributylstannyl)-1-ethoxyethylene, to join the two monomers. The final step involved the delicate closure of the hemiketal ring, which was accomplished by simply heating the intermediate to 120 °C, triggering a precise reaction that sealed the ring.
Following the successful synthesis of (–)-gukulenin A, the team created 15 additional derivatives to better understand the structural features contributing to its cytotoxic potency. Testing across four human cancer cell lines—lung, colon, leukemia, and ovarian—revealed that while the potency of gukulenin A varied by cancer type, derivatives with dimeric α-tropolone rings were consistently at least ten times more potent than their monomeric counterparts.
Implications for Cancer Research
The researchers propose that the enhanced cytotoxicity of these dimeric compounds may stem from α-tropolones’ affinity for divalent metals. This property could enable (–)-gukulenin A to bind to two separate metal-containing proteins simultaneously, offering insights into the compound’s mechanism of action. These findings not only deepen the understanding of (–)-gukulenin A’s effects but also set the stage for preclinical evaluations of its synthetic derivatives as potential anticancer agents.
The research was published in the journal Science and offers a promising pathway for future studies aimed at developing new treatments for ovarian cancer. The implications of this work extend beyond just one compound; they provide a framework for exploring the therapeutic potential of complex natural products that have long eluded synthetic chemists.
With the successful synthesis of (–)-gukulenin A, the prospects for expanding its therapeutic applications are more tangible than ever, marking a significant advancement in the fight against cancer.