A significant advancement in molecular engineering has the potential to increase the efficiency of light-driven chemical reactions by more than 40 times. A collaborative research team, led by Gordana Dukovic from the University of Colorado Boulder, alongside partners from the University of California Irvine and Fort Lewis College, has developed a method to minimize energy loss in tiny nanocrystals. Their findings were published in the journal Chem.
The researchers focused on addressing a critical challenge in the use of semiconductor nanocrystals for photocatalysis, a process that utilizes light to power chemical reactions. Traditionally, when these nanocrystals are exposed to light, they generate a fleeting burst of energy in the form of separated charges. Unfortunately, the electron and the corresponding positive charge, known as a “hole,” typically recombine quickly, leading to energy loss.
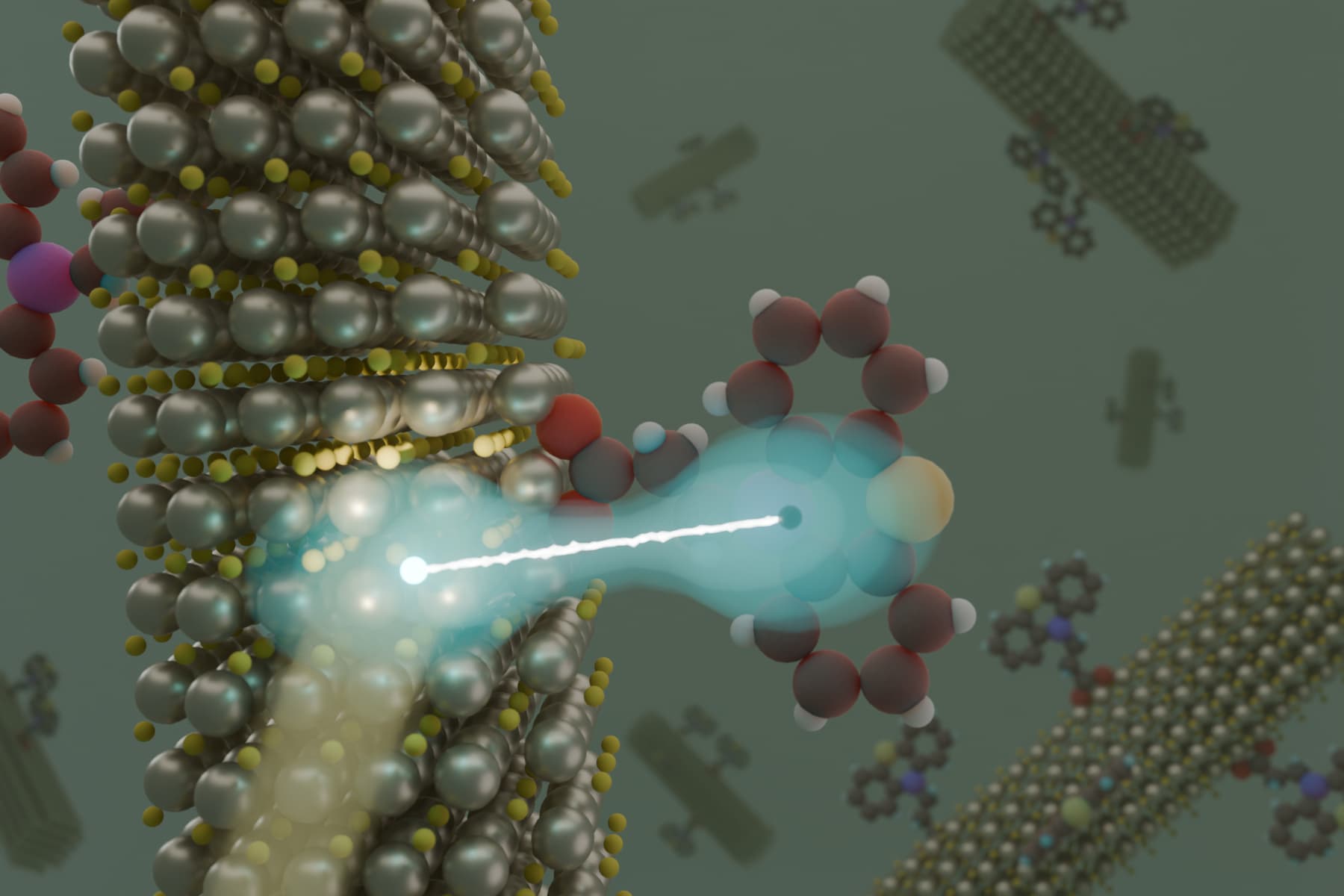
To combat this issue, the team engineered a molecule that effectively acts as a “molecular dam,” binding strongly to the nanocrystal’s surface. This design not only stabilizes the charge-separated state but also extends its duration to the longest recorded for these materials, lasting for microseconds. This extended timeframe allows researchers to harness the stored energy for practical chemical reactions.
The research primarily utilized cadmium sulfide (CdS) nanocrystals. The team created a phenothiazine derivative with two essential features: a carboxylate group that anchors the molecule to the nanocrystal surface, and a structure capable of quickly accepting the positive charge. By securing the molecule to the surface, the team successfully delayed the recombination of charges, leading to a more efficient energy storage system.
This work is part of the Energy Frontier Research Center (EFRC) funded by the U.S. Department of Energy, known as Ensembles of Photosynthetic Nanoreactors (EPN). EPN is a collaborative effort involving 17 senior investigators across nine universities and three U.S. national laboratories, designed to advance the frontiers of discovery in photochemical energy conversion.
The collaborative nature of this project allowed for an efficient exchange of ideas and rapid execution of experiments. Undergraduate researchers at Fort Lewis College, under the guidance of Kenny Miller, synthesized the key phenothiazine derivative. This compound was then sent to Jenny Yang’s group at UC Irvine for advanced electrochemical characterization, while Dukovic’s team at CU Boulder conducted further testing to assess compatibility and binding properties.
Dr. Sophia Click, a lead author of the study, expressed enthusiasm about the findings. “The first time I saw the results—how effective our ‘molecular dam’ was at slowing charge recombination—I knew we had struck gold,” she stated. The capability to extend charge separation from nanoseconds to microseconds could have profound implications for enhancing existing photocatalyst systems.
The development of this molecular dam could revolutionize the design of catalysts for light-driven chemistry. By improving the efficiency of the initial energy capture, this system enhances the overall process efficiency. The implications extend beyond a single reaction, potentially benefiting a wide array of light-driven chemical processes, including the production of chemical commodities and high-value chemicals.
This discovery in controlling charge separation at the nanoscale marks a pivotal step toward the future of light-driven chemical manufacturing. The vision of synthesizing materials such as plastics and pharmaceuticals through energy-efficient methods powered by light, rather than fossil fuels, is becoming more tangible.
While the full realization of this vision remains on the horizon, the collaboration’s work contributes significantly to the scientific understanding necessary for achieving these goals. The study, titled “Exceptionally Long-Lived Charge Separated States in CdS Nanocrystals with a Covalently Bound Phenothiazine Derivative,” highlights the potential for developing a robust and versatile chemical toolkit for future explorations in this field.
This research was supported by the U.S. Department of Energy’s Office of Science, as part of the EFRC EPN program, with additional experiments funded by the Air Force Office of Scientific Research.